The US Food and Drug Administration (FDA) has approved clinical trials for an implantable brain-computer interface (BCI).
Synchron, the developer of the brain computer interface "Stentrode," will begin a trial at Mount Sinai Hospital in New York later this year to test six subjects for early feasibility. In addition, Synchron will evaluate "safety and efficacy for patients with severe paralysis". If you look at the word brain computer interface, Engadget readers may wonder what Elon Musk founded Neuralink.
Neuralink announced the brain-embedded device Link in 2020, and in the spring of this year, it is appealing that technological development is steadily progressing, such as showing a video of the monkey who embedded it playing the game. I did.
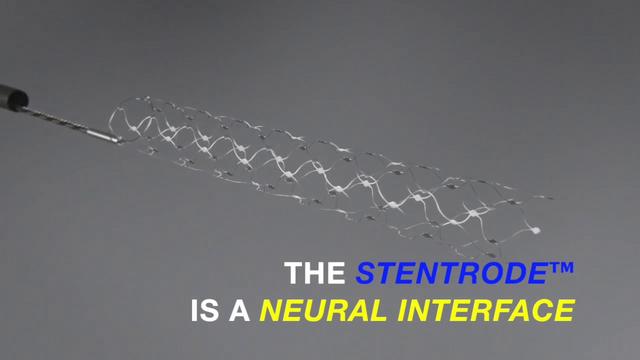
However, in order to sell a human-acting device such as BCI in the United States, it must be functionally and safely shown to the FDA and approved. Synchron, not Neuralink, was the first to reach clinical trials in the United States.
Synchron has already begun clinical trials of Stentrode in Australia ahead of the United States. We have implanted this device in four patients and have confirmed that it is possible to "transfer data from the motor cortex of the brain through electrodes and control digital devices."
And, according to a report in the Journal of NeuroInterventional Surgery, two of the subjects operated the computer by thinking only with their heads by transmitting brain impulses outside the body through the function of Stentrode, sending text messages, online banking, etc. It is said that it has become possible to shop online and perform work-related tasks. By the way, Stentrode implants require surgery that takes about 2 hours. This surgery is minimally invasive and requires a procedure in which the device is inserted into a blood vessel in the neck and then moved into the brain. Synchron states that Stentrode will be widely available in the medical setting in 3-5 years.
Source: Synchron
This content is not available in your privacy settings. Please change your settings here. This content is not available in your privacy settings. Please change your settings here. This content is not available in your privacy settings. Please change your settings here. This content is not available in your privacy settings.Please change the setting here